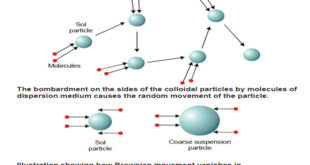
Kinetic Properties of Sols – In this topic, we will discuss Kinetic Properties of Sols. Brownian Movement – When a sol is examined with an ultramicroscope, the suspended particles are seen as shining specks of light. – By following an individual particle it is observed that the particle is undergoing …
Read More »Optical Properties of Sols
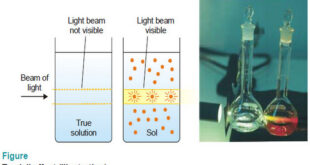
Optical Properties of Sols – In this topic, we will discuss Optical Properties of Sols as follow: Sols exhibit Tyndall effect Ultramicroscope shows up the presence of individual particles Sol particles can be seen with an Electron microscope (1) Sols exhibit Tyndall effect – When a strong beam of light …
Read More »Complex Splitting in ¹H NMR Spectra
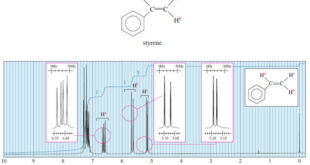
– In the last topic we talk about Spin-Spin Splitting in ¹H NMR Spectra, but In this topic, we will discuss The Complex Splitting. Complex Splitting in ¹H NMR Spectra – There are many cases of complex splitting, where signals are split by adjacent protons of more than one type, …
Read More »The Composition of Solutions During acid/Base Titration
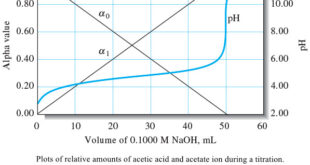
– In this topic, we will discuss The Composition of Solutions During acid/Base Titration. The Composition of Solutions During acid/Base Titration – We are often interested in the changes in composition that occur while a solution of a weak acid or a weak base is being titrated. – These changes …
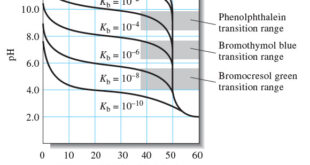
Read More »Titration Curves for Weak Bases
– In this topic, we will discuss The titration Curves for Weak Bases. Titration Curves for Weak Bases – The calculations needed to draw the titration curves for weak bases are analogous to those of a weak acid, as shown in The following Example . A 50.00-mL aliquot of 0.0500 …
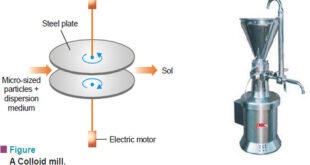
Read More »Preparation of Sols and Purification of Sols
– In this topic, we will discuss Preparation of Sols, Purification of Sols and its colors Preparation of Sols – Lyophilic sols may be prepared by simply warming the solid with the liquid dispersion medium e.g., starch with water. – On the other hand, lyophobic sols have to be prepared …
Read More »Titration Curves for Weak Acids
Titration Curves for Weak Acids – Four distinctly different types of calculations are needed to compute values for a weak acid (or a weak base) titration curves: (1) At the beginning, the solution contains only a weak acid or a weak base, and the pH is calculated from the concentration …
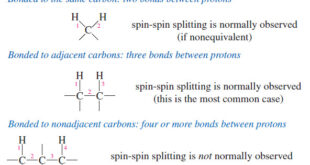
Read More »Spin-Spin Splitting in ¹H NMR Spectra
– In this topic, we will discuss The Spin-Spin Splitting in ¹H NMR Spectra. Theory of Spin-Spin Splitting – A proton in the NMR spectrometer is subjected to both the external magnetic field and the induced field of the shielding electrons. – If there are other protons nearby, their small …
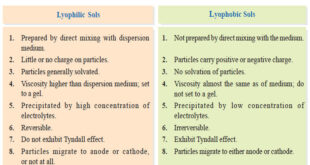
Read More »Lyophilic and Lyophobic sols : Defination, Properties, Comparison
– In this topic, we will discuss The Lyophilic and Lyophobic sols : Defination, Characteristics, and Comparison. Defination of Lyophilic and Lyophobic sols – Sols are colloidal systems in which a solid is dispersed in a liquid. – These can be subdivided into two classes : Lyophilic sols (solvent-loving) Lyophobic …
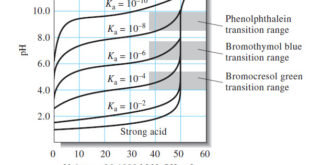
Read More »Titration of Strong Acids and Bases
– In this topic, we will discuss The Titration of Strong Acids and Bases. Introduction to Titration of Strong Acids and Bases – The hydronium ions in an aqueous solution of a strong acid have two sources: (1) the reaction of the acid with water. (2) the dissociation of water …
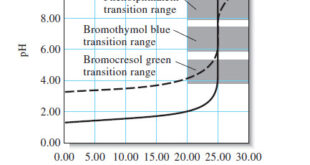
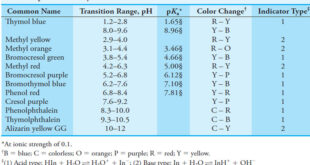
Read More »Indicators and Solutions for acid-base titration
– In this topic, we will discuss the Indicators and Solutions for acid-base titration. Indicators and Solutions for acid-base titration – Like all titrations, neutralization titrations depend on a chemical reaction of the analyte with a standard reagent. – There are several different types of acid/base titrations. – One of …
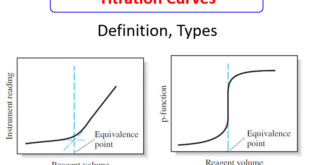
Read More »Titration Curves in Analytical Chemistry : Definition, Types
– In this topic, we will discuss the Titration Curves in Analytical Chemistry : Definition and Types Titration Curves – An end point is signaled by an observable physical change near the equivalence point of a titration. – The two most widely used signals involve (1) changes in color due …
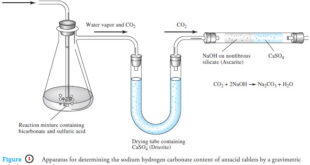
Read More »Gravimetric Titrations | Definition, Calculations & Advantages
Gravimetric titrations – Mass (weight) or gravimetric titrations differ from their volumetric counterparts in that the mass of titrant is measured rather than the volume. – Therefore, in a mass titration, a balance and a weighable solution dispenser are substituted for a buret and its markings. – Gravimetric titrations actually …
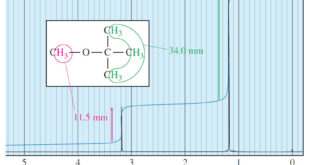
Read More »Areas of the Peaks in NMR Spectroscopy
– In this topic, we will discuss The Areas of the Peaks in NMR Spectroscopy Areas of the Peaks – The area under a peak is proportional to the number of hydrogens contributing to that peak. – For example, in the methyl tert-butyl ether spectrum (Figure 1), the absorption of …
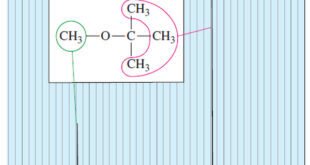
Read More »Number of Signals in NMR Spectroscopy
The Number of Signals – In general, the number of NMR signals corresponds to the number of different kinds of protons present in the molecule. – For example, methyl tert-butyl ether has two types of protons (Figure 1). – The three methoxy protons are chemically identical, and they give rise …
Read More »Colloids: Definition, History and Types
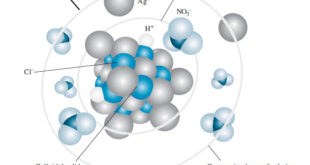
– In this topic, we will discuss the colloids: definition, History and Types History of colloids – Thomas Graham (1861) studied the ability of dissolved substances to diffuse into water across a permeable membrane. – He observed that crystalline substances such as sugar, urea, and sodium chloride passed through the …
Read More »Some Terms Used in Volumetric Titration
Some Terms Used in Volumetric Titration – A standard solution (or a standard titrant) is a reagent of known concentration that is used to carry out a volumetric titration. – The volumetric titration is performed by slowly adding a standard solution from a buret or other liquid dispensing device to …
Read More »Applications of Gravimetric methods
Applications of Gravimetric methods – Gravimetric methods have been developed for most inorganic anions and cations, as well as for such neutral species as water, sulfur dioxide, carbon dioxide, and iodine. – A variety of organic substances can also be determined gravimetrically. – Examples include lactose in milk products, salicylates …
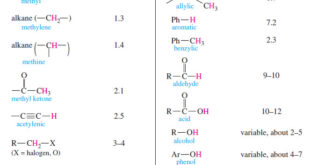
Read More »Chemical Shift in NMR Spectroscopy
– In this topic, we will discuss the Chemical Shift in 1H NMR Spectroscopy. What is Chemical Shift? – The variations in the positions of NMR absorptions, arising from electronic shielding and deshielding, are called chemical shifts. – Chemical shift is The difference (in parts per million) between the resonance …
Read More »Precipitation Gravimetry
– In this topic, we will discuss the Precipitation Gravimetry as an important one of Gravimetric Analysis in analytical chemistry. What is Gravimetric Analysis? – Gravimetric analysis is a method to determine the quantity of an analyte based on the mass of a solid. – Gravimetric methods of analysis are …
Read More » Read Chemistry
Read Chemistry